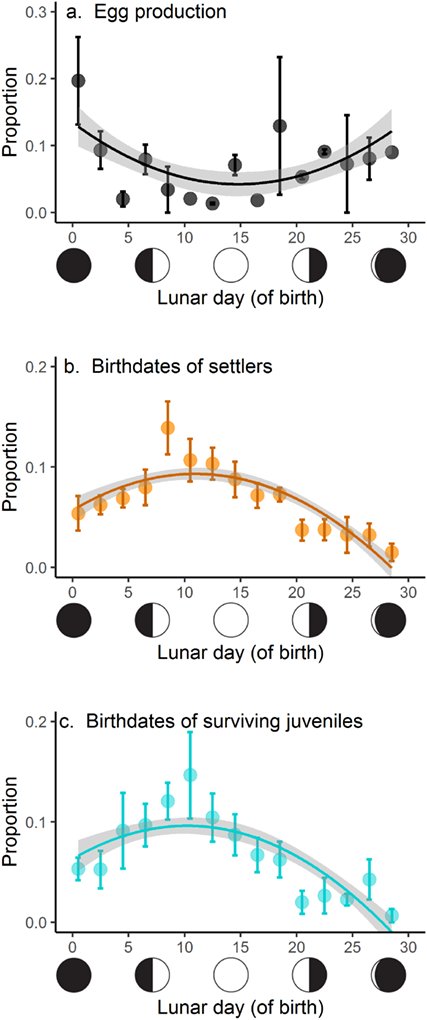
 Team Thalassoma's second paper on moonlight and reproduction and recruitment patterns in T. hardwicke led by Captain Jeff S. Shima is out in Ecology (pdf). Abstract: Most organisms reproduce in a dynamic environment, and life‐history theory predicts that this can favor the evolution of strategies that capitalize on good times and avoid bad times. When offspring experience these environmental changes, fitness can depend strongly upon environmental conditions at birth and at later life stages. Consequently, fitness will be influenced by the reproductive decisions of parents (i.e., birth date effects) and developmental decisions (e.g., adaptive plasticity) of their offspring. We explored the consequences of these decisions using a highly iteroparous coral reef fish (the sixbar wrasse, Thalassoma hardwicke ) and in a system where both parental and offspring environments vary with the lunar cycle. We tested the hypotheses that (1) reproductive patterns and offspring survival vary across the lunar cycle and (2) offspring exhibit adaptive plasticity in development time. We evaluated temporal variation in egg production from February to June 2017, and corresponding larval developmental histories (inferred from otolith microstructure) of successful settlers and surviving juveniles that were spawned during that same period. We documented lunar‐cyclic variation in egg production (most eggs were spawned at the new moon). This pattern was at odds with the distribution of birth dates of settlers and surviving juveniles—most individuals that successfully survived to settlement and older stages were born during the full moon. Consequently, the probability of survival across the larval stage was greatest for offspring born close to the full moon, when egg production was at its lowest. Offspring also exhibited plasticity in developmental duration, adjusting their age at settlement to settle during darker portions of the lunar cycle than expected given their birth date. Offspring born near the new moon tended to be older and larger at settlement, and these traits conveyed a strong fitness advantage (i.e., a carryover effect) through to adulthood. We speculate that these effects (1) are shaped by a dynamic landscape of risk and reward determined by moonlight, which differentially influences adults and offspring, and (2) can explain the evolution of extreme iteroparity in sixbars.
Team Thalassoma's second paper on moonlight and reproduction and recruitment patterns in T. hardwicke led by Captain Jeff S. Shima is out in Ecology (pdf). Abstract: Most organisms reproduce in a dynamic environment, and life‐history theory predicts that this can favor the evolution of strategies that capitalize on good times and avoid bad times. When offspring experience these environmental changes, fitness can depend strongly upon environmental conditions at birth and at later life stages. Consequently, fitness will be influenced by the reproductive decisions of parents (i.e., birth date effects) and developmental decisions (e.g., adaptive plasticity) of their offspring. We explored the consequences of these decisions using a highly iteroparous coral reef fish (the sixbar wrasse, Thalassoma hardwicke ) and in a system where both parental and offspring environments vary with the lunar cycle. We tested the hypotheses that (1) reproductive patterns and offspring survival vary across the lunar cycle and (2) offspring exhibit adaptive plasticity in development time. We evaluated temporal variation in egg production from February to June 2017, and corresponding larval developmental histories (inferred from otolith microstructure) of successful settlers and surviving juveniles that were spawned during that same period. We documented lunar‐cyclic variation in egg production (most eggs were spawned at the new moon). This pattern was at odds with the distribution of birth dates of settlers and surviving juveniles—most individuals that successfully survived to settlement and older stages were born during the full moon. Consequently, the probability of survival across the larval stage was greatest for offspring born close to the full moon, when egg production was at its lowest. Offspring also exhibited plasticity in developmental duration, adjusting their age at settlement to settle during darker portions of the lunar cycle than expected given their birth date. Offspring born near the new moon tended to be older and larger at settlement, and these traits conveyed a strong fitness advantage (i.e., a carryover effect) through to adulthood. We speculate that these effects (1) are shaped by a dynamic landscape of risk and reward determined by moonlight, which differentially influences adults and offspring, and (2) can explain the evolution of extreme iteroparity in sixbars.